By Dr. Rajesh Bollam
February 12, 2026
CAR-T Cell Therapy Beyond Leukemia: What’s Next in Cancer Treatment?
- CAR-T delivers >80% remission rates in relapsed B-cell ALL, transforming leukemia outcomes.
- Now approved for lymphoma and multiple myeloma, with 50–90% response rates in advanced disease.
- Over 1,000 global clinical trials are expanding CAR-T into solid tumours.
- Next-gen innovations include dual-target, off-the-shelf, and safer engineered CAR-T designs.
- Cost and infrastructure remain barriers, but scalability is improving.
- India is emerging in CAR-T development, expanding advanced cellular therapy access.
- CAR-T is shifting from last-line rescue to a core precision oncology strategy.
What is CAR-T Cell Therapy
Revolutionary Next Step in Cancer with CAR-T Cell Therapy
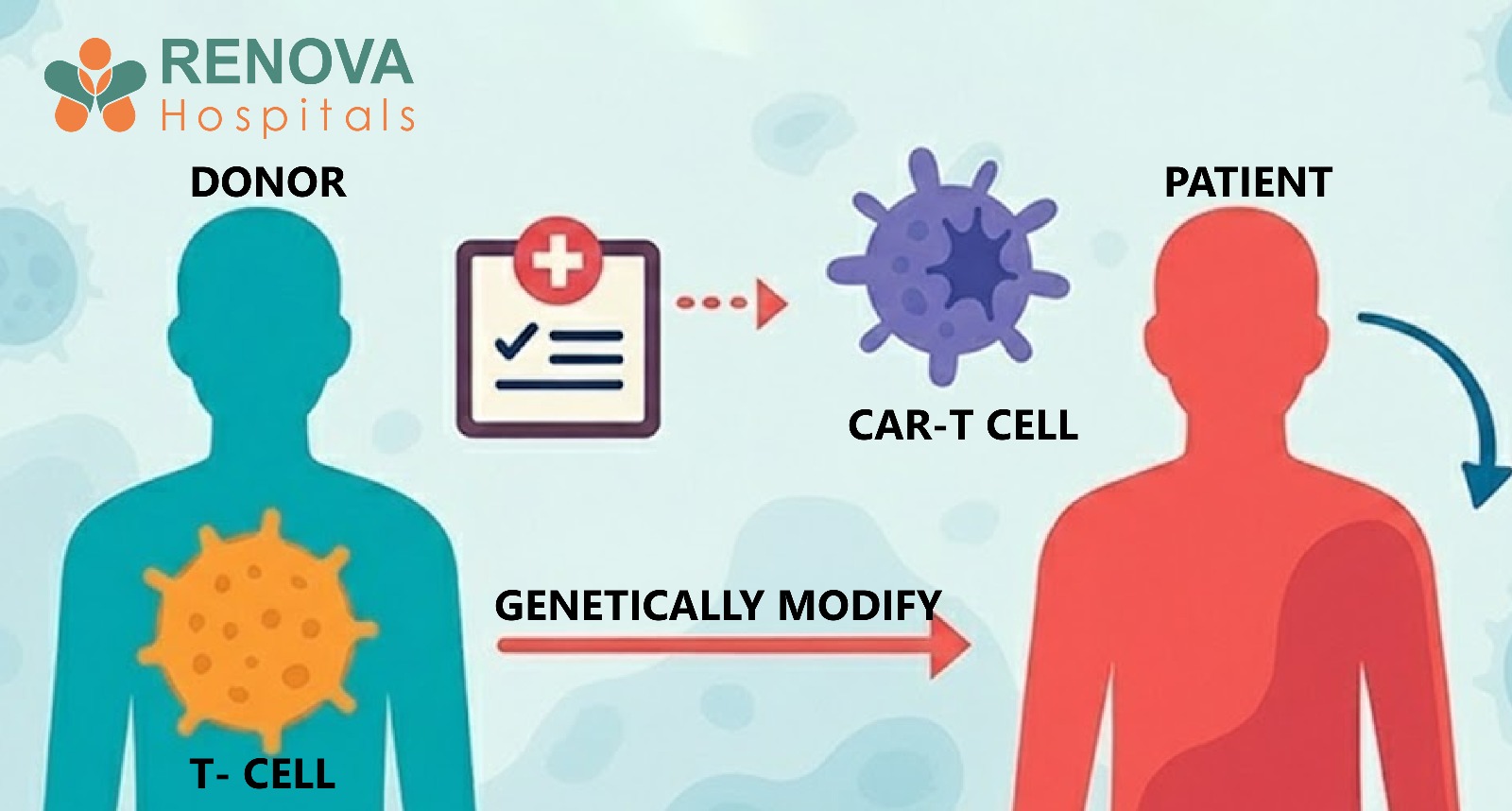
How CAR-T Therapy Works
- T-cells are collected from the patient through leukapheresis
- These cells are genetically engineered in a laboratory to express a CAR receptor
- The CAR receptor is designed to recognise a specific protein on cancer cells
- Modified cells are multiplied in large numbers
- After lymphodepleting chemotherapy, the cells are infused back into the patient
- The engineered cells seek and destroy cancer cells
Proven Success in Leukemia: The Foundation
- Complete remission rates in pediatric and young adult relapsed ALL exceeded 80%
- Many patients had exhausted all other treatment options
- Durable remissions were observed in a significant percentage
Why Leukemia Was Ideal for CAR-T
Leukemia provided a strong starting point because:
- Cancer cells circulate in the blood and the bone marrow (easier immune access)
- CD19 is a clear and consistent target
- Tumour burden can be rapidly reduced
Expansion Beyond Leukemia: Current Approved Indications
- Overall response rates of 50–80% in diffuse large B-cell lymphoma (DLBCL)
- Durable complete remission in many patients
- Improved survival compared to historical outcomes
- Using CAR-T earlier (second-line instead of third-line)
- Reducing relapse rates
- Managing resistance mechanisms
- Optimising long-term immune persistence
- Response rates exceeding 70–90% in heavily pretreated patients
- Deep MRD-negative remissions
- Prolonged progression-free survival
- It is highly expressed on malignant plasma cells
- Has limited expression in normal tissues
- Provides a relatively safe therapeutic window
- Median progression-free survival exceeding 12 months in advanced cases
- Significant symptom improvement
- Reduced tumour burden even after multiple prior therapies
- CAR-T as second-line therapy
- CAR-T in newly diagnosed high-risk patients
Solid Tumours: The Most Ambitious Frontier
- Physical barriers preventing immune cell infiltration
- Immunosuppressive tumour microenvironments
- Heterogeneous antigen expression
- Risk of damaging normal tissues
- Glioblastoma
- Ovarian cancer
- Pancreatic cancer
- Lung cancer
- Hepatocellular carcinoma
- Targeting HER2, GD2, EGFRvIII
- Combining CAR-T with checkpoint inhibitors
- Engineering CAR-T cells resistant to tumour suppression
Next-Generation CAR-T Innovations
- Recognise two tumour markers
- Reduce relapse probability
- Improvethe durability of response
Current CAR-T manufacturing takes 2–4 weeks.
- Patients may require bridging therapy
- The disease may progress
- Immediate availability
- Standardized production
- Reduced cost over time
- Cytokine Release Syndrome (CRS)
- Immune effector cell-associated neurotoxicity syndrome (ICANS)
- Built-in safety switches
- Tunable CAR signaling
- Reduced cytokine intensity engineering
- Use in older patients
- Outpatient administration in future
- Broader patient eligibility
CAR-NK and Other Cellular Platforms
- Natural Killer (NK) cells
- Macrophages
- Gamma-delta T cells
- Lower risk of graft-versus-host disease
- Reduced neurotoxicity
- Easier mass production
Cost, Accessibility, and Global Expansion
- $350,000–$475,000 per infusion (excluding hospitalisation)
- Complex manufacturing
- Limited certified centres
- ICU-level monitoring
- High upfront cost
- India reports over 1.4 million new cancer cases annually
- Hematologic malignancies remain a substantial burden
- Developing indigenous CAR-T platforms
- Reducing manufacturing costs
- Expanding tertiary oncology centres
- “CAR-T therapy cost in India”
- “Is CAR-T available near me?”
- “Best hospital for CAR-T in Hyderabad or Delhi?”
What the Future Holds: 5–10 Year Outlook
- Earlier integration into treatment algorithms
- Combination with immunotherapy drugs
- Outpatient CAR-T models
- Expanded solid tumour success
- Reduced costs through automation
- Experimental therapy To Core oncology strategy
The Future of CAR-T Is Already Taking Shape
Category
- Joint Replacement
- Joint Replacement
- Joint Replacement